Contact
Positions
Professor
- Organization:
- West Virginia University School of Medicine
- Department:
- Biochemistry and Molecular Medicine
- Classification:
- Faculty
Member
- Organization:
- West Virginia University WVU Cancer Institute
- Department:
- WVU Cancer Institute Research Programs
- Classification:
- Faculty
Publications
1. Khramtsov, V.V. 2021, Spin Probes and Imaging Using Nitroxides, In: Nitroxides: Synthesis, Properties and Applications, Edited by O. Ouari, D. Gigmes, The Royal Society of Chemistry, London, UK, p.p. 147-186.
2. Velayutham M, Poncelet M, Eubank TD, Driesschaert B and Khramtsov VV. Biological Applications of Electron Paramagnetic Resonance Viscometry Using a 13C-Labeled Trityl Spin Probe. Molecules 2021, 26(9), 2781
3. Dzikovski B, Khramtsov VV, Chandrasekaran S, Dunnam C, Shah M, Freed JH. 2020, Microsecond Exchange Processes Studied by Two-Dimensional ESR at 95 GHz, JACS, 142, 21368−21381.
4. Poncelet M, Driesschaert B, Tseytlin O, Tseytlin M, Eubank TD, Khramtsov VV. 2019, Dextran-conjugated triarylmethyl radicals as biocompatible spin probes for EPR spectroscopy and imaging, Med.Chem.Lett. 29, 1756-1760.
5. Gorodetskii AA, Eubank TD, Driesschaert B, Poncelet M, Ellise E, Khramtsov VV, Bobko AA. 2019, Multiparametric Overhauser-enhanced magnetic resonance imaging: visualization of interstitial oxygenation, acidosis and inorganic phosphate concentration, Sci Rep. 9 (1), 12093.
6. Tseytlin O, Guggilapu P, Bobko AA, AlAhmad H, Xu X, Epel B, O’Connell R, Hoblitzell EH, Eubank TD, Khramtsov VV, Driesschaert B, Kazkaz E, Tseytlin M. 2019, Modular Imaging System: Rapid Scan EPR at 800 MHz, J. Magn. Reson, 305, 94-103..
7. Khramtsov VV. 2018, In vivo molecular EPR-based spectroscopy and imaging of tumor microenvironment and redox using functional paramagnetic probes, Antiox. Redox Signaling. 28 (15), 1365-1377.
8. Bobko AA, Eubank TD, Driesschaert B, and Khramtsov VV. 2018, "In Vivo EPR Assessment of pH, pO2, Redox Status and Concentrations of Phosphate and Glutathione in Tumor Microenvironment," J. Vis. Exp. (133), e56624; https://www.jove.com/video/56624 .
9. Khramtsov VV. 2018, In Vivo EPR: Radical Concepts for Translation to the Clinical Setting, Antiox. Redox Signaling, 28 (15), 1341-1344.
10. Poncelet M, Driesschaert B, Bobko AA & Khramtsov VV. 2018 Triarylmethyl-based biradical as a superoxide probe, Free Radical Research, 52 (3), 373-379.
11. Bobko AA, Eubank TD, Driesschaert B, Dhimitruka I, Evans J, Mohammad R, Tchekneva EE, Dikov MM and Khramtsov VV. 2017, "Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression," Sci.Rep. 7, 41233; www.nature.com/articles/srep41233.
12. Khramtsov VV, Bobko AA, Tseytlin M, and Driesschaert B. 2017, Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment, Analyt. Chem. 89 (9), 4758–4771.
13. Bobko AA, Evans J, Denko NC, and Khramtsov VV. 2017. Concurrent Longitudinal EPR Monitoring of Tissue Oxygenation, Acidosis, and Reducing Capacity in Mouse Xenograft Tumor Models, Cell Biochem. Biophys. 75: 247-253.
Research Program
Magnetic Resonance Approaches to Biomedicine
Research Interests
Supported by NIH R01EB014542 (04.2012-03.2016), R01CA194013 (04.2015-03.2020), R01CA192064 (08.2015-07.2020)
Most of my research is devoted to the development and application of new magnetic resonance approaches to biomedicine, including electron paramagnetic resonance (EPR) spectroscopy and imaging and Overhauser-enhanced magnetic resonance imaging (OMRI or proton-electron double-resonance imaging, PEDRI). Our current projects develop the unique paramagnetic probes allowing noninvasive simultaneous detection of tissue oxygenation, acidity (pH), redox status, intracellular glutathione (GSH) and interstitial inorganic phosphate in living subjects. Based on thorough examination of recent literature and experimental data accumulated in our laboratories, we published a new hypothesis of a Janus-faced tumor microenvironment (TME) and its role in tumorigenesis.
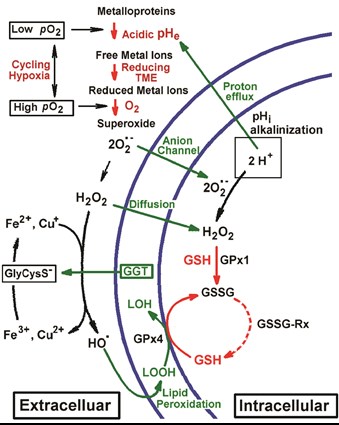
Janus-faced tumor microenvironment. The key components of the chemical TME, oxygen, pH, redox and GSH, are shown in red. Hypoxia-induced acidosis potentiates accumulation of free metal ions such as Fe3+ and Cu2+. In its turn, a high reducing capacity of TME promotes metal ion reduction to Fenton-active state, e.g. via γ‐glutamyltransferase (GGT)/GSH-dependent generation of reducing cysteinyl-glycine dipeptide, GlyCysS¯. A cycling local hypoxia in TME facilitates further electron transfer to oxygen with formation of O2•- radical, triggering radical reaction cascade of O2•- dismutation to H2O2 followed by OH-radical formation via Fenton reaction. Low-reactive ROS, H2O2 and O2•-, penetrate into tumor cells by diffusion or via anion channels, latter contributing to increase of intracellular pH (pHi) with corresponding decrease in oxidizing potential of ROS. In a contrary, low acidic pHe in TME enhances ROS oxidizing potential towards surrounding cells that may result in oxidative damage and mutagenesis as well as adaptive response (e.g., increase of GSH) and changing cellular phenotype. Increase in H2O2, O2•-, GSH and pH has been shown contribute into the triggering cells in proliferation stage. Compared to normal cells, cancer cells are well protected against oxidative stress, in part by elevated GSH content and activities of GSH-dependent antioxidant enzymes, including glutathione peroxidases (GPx1 and GPx4 denote peroxidases that target hydrogen peroxide and lipid peroxide, correspondingly) and glutathione reductase, GSSG-Rx (Khramtsov & Gillies, 2014, ARS, 21, 723–729).
The focus of the current NIH-supported research is application of multifunctional in vivo TME analysis using in vivo magnetic resonance spectroscopy and imaging to investigate TME role in tumor progression and therapy.