Contact
About Robert Goodman
1980-present: faculty member Department of Physiology (& Pharmacology), WVU Sabbatical leaves in Cambridge UK (1989), Nouzilly FR (1997), Melbourne AU (2007)
Positions
Professor
- Organization:
- West Virginia University School of Medicine
- Department:
- Physiology, Pharmacology & Toxicology
- Classification:
- Faculty
Professor
- Organization:
- West Virginia University School of Medicine
- Department:
- Department of Neuroscience
- Classification:
- Faculty
Professor
- Organization:
- West Virginia University School of Medicine
- Department:
- Rockefeller Neuroscience Institute (SOM)
- Classification:
- Faculty
Education
- PhD, University of Pittsburgh
Publications
Goodman RL, Inskeep EI 2006 Neuroendocrine Control of the Ovarian Cycle of the Sheep. In: Knobil and Neill’s Physiology of Reproduction, Third Edition (Neill JD, ed) , Elsevier, Amsterdam pp. 2389-2447
Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Alain Caraty A, Ciofi P, Clarke IJ 2007 Kisspeptin neurons in the arcuate nucleus of the ewe also express dynorphin A and neurokinin B. Endocrinology 148: 5752-5760
Bogusz AL, Hardy SL, Lehman MN, Connors JM, Hileman SM, Sliwowska JH, Billings HJ, McManus CJ, Valent M, Singh SR, Nestor CC, Coolen LM, Goodman RL 2008 Evidence that -amino butyric acid is part of the neural circuit mediating estradiol negative feedback in anestrous ewes. Endocrinology 149:2762-2772
Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN 2009 Kisspeptin and seasonality in sheep. Peptides 30: 154-163 PMID: 18838092
Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN 2010 The kisspeptin /neurokinin B/dynorphin (KNDY) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology 151: 301-311 PMID: 19880810
Dupre SM, Miedzinska K, Duval CV, Yu L, Goodman RL, Lincoln GA, Davis JR, McNeilly AS, Burt BD, Loudon A 2010 Long day response in seasonal mammals, identification of a conserved Eya3 signal, and prolactin releasing factors. Curr Biol 20: 829-835 PMID: 20434341
Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN 2010 Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol 22: 674-681 PMID: 20456601
Lehman MN, Coolen LM, Goodman RL 2010 KNDy (kisspeptin/neurokinin B/dynorphin) cells of the arcuate nucleus: a central node in the control of GnRH secretion Endocrinology 151: 3479-3489 PMID: 20501670
Lehman MN, Merkley CM, Coolen LM, Goodman RL 2010 Anatomy of the kisspeptin neural network in mammals. Brain Res 1364: 90-102 PMID: 20858464
Lehman MN, Ladha Z, Coolen LM, Hileman SM, Connors JM, Goodman RL 2010 Neuronal Plasticity and seasonal reproduction in sheep. Eur J Neurosci 32:2152-2164.PMID: 21143669
Awards
1992: Benedum Distinguish Scholar
About Robert Goodman
My research career has focused on the neuroendocrine mechanisms by which the ovarian steroids, estradiol (E2) and progesterone, control secretion of gonadotropin-releasing hormone (GnRH). GnRH is the major signal from the brain that controls secretion of gonadotropins from the anterior pituitary and thus ovarian function. The feedback actions of ovarian steroids are complex, but critical for the control of the ovarian cycle and for the physiological shut-down of fertility that occurs prior to puberty and annually in seasonal breeders. Our work is currently focused on two neuroendocrine mechanisms critical for the events of the ovarian cycle: 1) the generation and negative feedback control of episodic GnRH secretion, and 2) the positive feedback action of E2 that induces the preovulatory GnRH surge and ovulation of a mature ovum. Throughout most of the cycle, GnRH secretion is secreted episodically, rather than continuously, a pattern that is required for normal gonadotropin secretion from the pituitary. Our work on this system identified a set of neurons in the arcuate nucleus that produce three neuropeptides: kisspeptin, neurokinin B (NKB), and dynorphin, known as KNDy neurons. Kisspeptin and NKB are essential for fertility in humans and stimulate GnRH release, while dynorphin is an inhibitory endogenous opioid peptide. The discovery of KNDy neurons, together with information on neurons containing the receptors for these peptides, led to development for a model for the synchronization of GnRH neural activity necessary for episodic GnRH secretion and to evidence that dynorphin and kisspeptin release from KNDy neurons mediates the negative feedback actions of progesterone and E2 respectively. Evidence is also beginning to support important roles for kisspeptin and NKB in positive feedback E2, and this has recently become an area of focus for our research.
Research Program
Neuroendocrine control of gonadotropin secretion; mechanisms responsible for seasonal reproductive function and onset of ovarian cycles at puberty.
Research Interests
Our primary research interests are:
1) the neural mechanisms controlling episodic secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus during the normal ovarian cycle and 2) the structural and functional changes in the hypothalamus responsible for the reversible suppression of GnRH secretion that occurs prior to puberty and annually in seasonal breeders. Our work in the first area is focused on the role of endogenous opioid peptides (EOP). We are currently testing the hypothesis that one EOP, dynorphin, mediates the inhibition of GnRH by progesterone and determining the neural substrates responsible for this inhibition. In the second area, we have identified a key neural circuit that inhibits GnRH secretion and is active in anestrous, but not in breeding season, ewes. We are now examining possible anatomical or functional alterations that underlie the seasonal changes in activity of this system and are exploring the mechanisms by which two hormones, melatonin and thyroid hormones, influence these alterations. We employ a variety of whole animal procedures (including neurosurgical procedures, collection of CSF and hypophyseal portal blood), histological techniques (e.g. confocal analysis of dual and triple label immunocytochemically identified proteins) and molecular approaches (e.g. in situ hybridization, administration of antisense oligonucleotides).
GnRH Secretion
What structural and functional changes occur in the hypothalamus that GnRH secretion, and hence ovarian function, annually in seasonal breeders?
We have identified a key neural circuit that inhibits GnRH secretion and is active in non-breeding season (anestrus), but not in breeding season, ewes. The central players in this circuit is a group of dopaminergic (DA) neurons that are stimulated by the ovarian hormone, estradiol, in anestrus, but not the breeding season and inhibit GnRH release. Although these neurons respond to estradiol they do not contain estrogen receptors, so estrogen-responsive afferents must be involved. We have identified the anatomical location of these afferents to the DA neurons and are currently determining their phenotype. We are also examining possible anatomical or functional alterations that underlie the seasonal changes in activity of this system and are exploring the mechanisms by which two hormones, melatonin and thyroid hormones, influence these alterations.
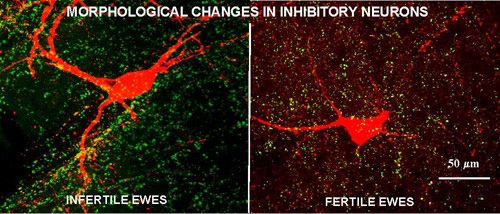
Plasticity in the Hypothalamus
What are the neural mechanisms controlling episodic secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus during the normal ovarian cycle?
Our recent work in this area has been focused on the role of endogenous opioid peptides (EOP) in mediating the negative feedback action of progesterone. A number of lines of evidence have implicated one EOP, dynorphin, mediating this action of progesterone and we have identified three sub-populations of dynorphin neurons as likely candidates because they contain progesterone receptors. We are currently determining which of these sub-populations are involved. This work has also expanded to examination of the role of two additional peptides: neurokinin B (NKB) and orphanin FQ (OFQ). NKB is of interest because it is also found in one sub-population of dynorphin neurons and OFQ because it is found in almost all GnRH neurons. We have begun to characterize the effects of these peptides on LH secretion and to determine where their receptors are located.